In a feat of biomedical engineering, researchers create model embryos that could be used to study the mysterious stages of early human development.
1:00 PM
Author |
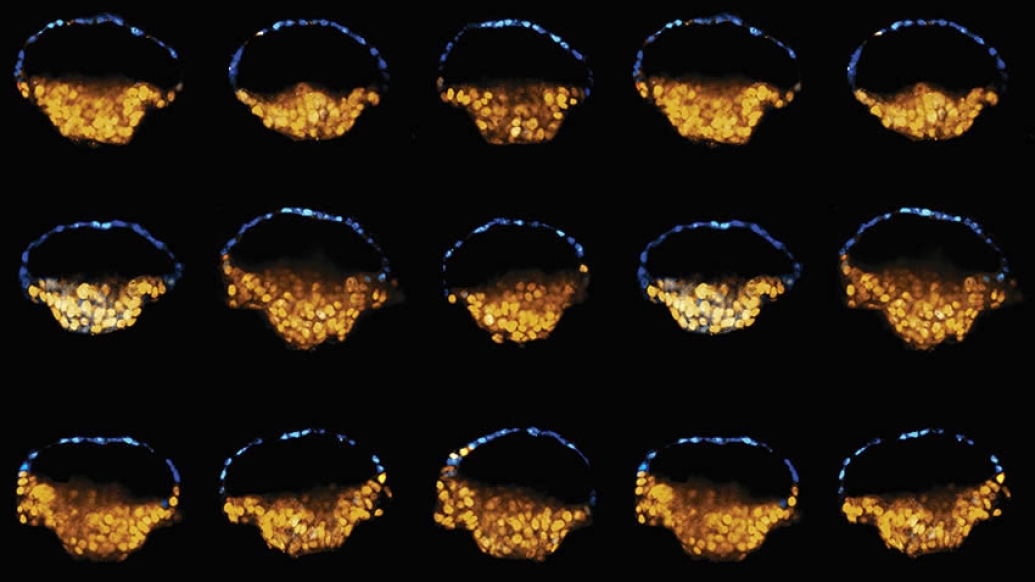
In a new landmark study published in Nature, co-senior authors Jianping Fu, Ph.D., associate professor of mechanical and biomedical engineering and cell and developmental biology and Deborah Gumucio, Ph.D., professor emerita of cell and developmental biology, and their team have developed a new way to reliably create stem cell colonies that resemble parts of newly implanted human embryos. These structures, which many refer to as embryoids, can be generated from induced pluripotent stem cells (iPSC), cells reprogrammed from adult tissues that behave like human embryonic stem cells (hESC), which have the power to become any type of cell in the human body.
Fu and Gumucio described what they did and what this work means for the future of research into human development and reproduction. Their answers have been edited for length and clarity.
How does this work build on your earlier work on embryoids?
Fu: Deb and I previously collaborated on a paper describing how we could use pluripotent stem cells to generate cysts that could develop into amnion tissues and a second paper that reports how, in rare cases, those multicellular cysts adopt an asymmetric structure that resembles part of the implanting human embryo.
After these initial, exciting observations, we started thinking about how we could leverage an engineering tool called microfluidics, which allows us to control fluids in miniature channels. This technique provided culture environments for pluripotent stem cells to generate these embryo-like structures more reproducibly and controllably. With our new system, we can now create these embryoid structures more than 90 percent of the time.
Our model also revealed a new surprise: within the asymmetric cysts, we observed primordial germ cells (PGCs), the precursors for sex cells. Previous studies in other species had suggested that PGCs develop just after implantation, but this work was the first demonstration of that process using human cells.
Gumucio: The previous culture system involved a bulk culture of many cysts; we didn't know which ones would eventually make fully amnion cysts and which ones would generate one of these really interesting looking asymmetric cysts. Then, we found that by varying the signaling molecules present in the culture, we could make more of the asymmetric embryo-like cysts. We therefore turned to microfluidics to control the signaling more precisely.
[We] have a reproducible system that could potentially be used to diagnose causes of infertility or screen for drugs and chemicals that cause early embryonic toxicity. There's enough to keep us busy for decades.Deborah Gumucio, Ph.D.
Our new system traps little balls of cells in a microfluidic chamber in such a way that you can apply chemicals or signals to cells only on one side of the ball. The microfluidic delivery also allows control over the timing of the signal.
So you take all the randomness out, you control time and space and get it all reproducible. That's what you need to develop a high throughput system for basic research and screening applications.
What does the generation of these structures allow you and other researchers to investigate?
Gumucio: If we kind of back up to the 10,000-foot view, the whole process of human embryo development during and shortly after implantation, from about two weeks to six weeks or so, is a total black box. We can't see it, it's too small to image and it would be totally unethical to harvest it to take a look at it. Having an in vitro system like this allows us to replicate at least some of the events that happen during implantation.
Fu: A lot of fundamental questions about human development can be studied using this microfluidic system. For example, what signals trigger embryonic cells to differentiate and organize during development? What are the roles of geometry and forces in embryonic development? Why do certain cells differentiate into one cell type while others choose another differentiation path? The controllability and scalability of this microfluidic system also make it ideal for screening applications.
Gumucio: We can look at the signaling cascade that leads to amnion cells, for example. There are maladies where amnion doesn't form or grow properly, or is too weak; we can learn why and perhaps correct it. We can now also research how PGCs arise and how gastrulation (the phase during early development where a single layer of cells becomes a multilayered structure known as the gastrula) is triggered and how pluripotent cells first respond to that trigger. All that on top of being able to have a reproducible system that could potentially be used to diagnose causes of infertility or screen for drugs and chemicals that cause early embryonic toxicity, drugs that pregnant women would want to take. There's enough to keep us busy for decades.
Do these structures eliminate the need to use stem cells from donated embryos to do research?
Gumucio: We're talking, in a way, about three different ways to go about human development research. One is to take an embryo donated after IVF and culture it in a lab for research. That's been done by a few groups, but the asymmetric cyst structures never formed, and there are ethical limits to the extent to which you can culture whole intact embryos. The second strategy is to use established lines of hESC and the third is to generate iPSCs from adult human tissues.
With the established hESC lines, you have a major advantage in that you can do the same experiment over and over again with cells from the same genetic background, which is great for exploring how a system is working. But humans are obviously not genetically identical. With iPSCs, you get to factor in the whole business of genetic variability. You can do experiments with iPSCs from different people and say which of the steps we see are variable and which are not; it's a window into what's at the core of the process and what genetic variability is doing to the system.
So you could study infertility by taking skin cells from a couple and looking at those in your system?
Gumucio: Exactly. We would take skin cells separately from male and female and create iPSCs and we'd be looking for a dominant defect in amnion generation or asymmetric cyst formation. This could get us a whole lot of information that we don't have.
Fu: The key is now we can study this black box of human development using patient-derived iPSCs to understand infertility. This microfluidic system offers the first human-relevant experimental model to study human development and reproduction in a very controllable and scalable fashion.
Did your team have any bioethical concerns in the generation of these structures?
Fu: We stopped these experiments after 3-4 days. We understand there are sensitive issues here.
Gumucio: When we started this work, we were just trying to make amnion; everything else was a complete surprise. We were surprised that some of the cysts were asymmetric and that PGCs emerged and that epiblast cells on the other side of the cysts started to undergo gastrulation-type movements. Together, these processes resemble the early events of embryo development.
But there are very critical things missing in our system, including several cell types that contribute to formation of the placenta for example. Also missing is the uterus and its blood supply and nutrients, without which nothing else will really happen. Essentially, our system is a kind of organoid system similar to what others have created to study kidneys, eyes, guts and even brains. It's clearly not a whole embryo.
We did consider the Warnock rule, an international agreement regarding culturing human embryos that was established in 1984 by a group of scientists, ethicists, lawyers, religious leaders and others. The Warnock compromise holds that such cultures should be destroyed 14 days after fertilization or at the first sign of the primitive streak (the structure that signals that gastrulation is beginning).
However, our organoids are not formed by fertilization and they are not whole embryos. It's also unclear how the culture timing (3-4 days) relates back to actual embryo development (14 days). Nevertheless, our structures do seem to initiate gastrulation-like movements, though this process is very disorganized, producing by four days a chaotic mass of cells, rather than an embryo-like entity. Does this cross the Warnock line? Clearly, scientists need to carefully consider the ethical questions generated by these and other new studies as a group and establish some guidelines. Jianping is working with other colleagues to look at these issues very closely.
Fu: Many of us involved in this new research area are in close conversations with ethics experts, talking about the potential concerns or sensitivities. Many researchers are trying to provide guidelines to think about what are the boundaries, such as embryo-like structures should not be used for reproductive purposes. As Deb mentioned, this 14-day Warnock agreement and how it should be interpreted in light of these new studies needs to be clarified.
In addition to senior authors Deborah Gumucio, Ph.D. and Jianping Fu, Ph.D., the paper includes the following U-M authors: Yi Zheng, Ph.D.; Xufeng Xue, Ph.D.; Yue Shao, Ph.D.; Sicong Wang, Ph.D.; Sajedeh Nasr Esfahani, Ph.D.; Zida Li, Ph.D., all with U-M's Integrated Biosystems and Biomechanics Laboratory.
Papers cited:
-
"Controlled modelling of human epiblast and amnion development using stem cells." Nature. DOI: 10.1038/s41586-019-1535-2
-
"Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche." Nature Materials, volume16, pages 419–425 (2017). DOI: 10.1038/nmat4829
-
"A pluripotent stem cell-based model for post-implantation human amniotic sac development." Nature Communications, volume 8, Article number: 208 (2017). DOI: 10.1038/s41467-017-00236-w

Explore a variety of health care news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!