Could a tiny device designed to avert stroke-provoking clots be a substitute for medication? The technology is an option for certain at-risk patients.
8:00 AM
Author |
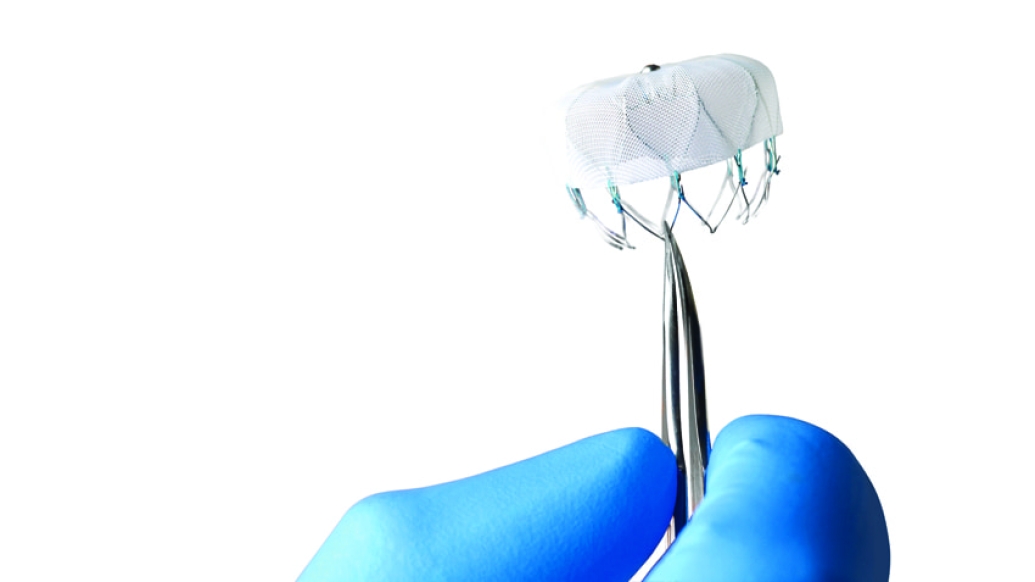
For certain non-valvular atrial fibrillation patients who do not respond well to (or are at risk from) taking warfarin, a lightweight heart implant the size of a quarter has been hailed as a feasible alternative.
In recent trials, researchers found a clot-stopping device known as the Watchman was comparable to warfarin in offering effective protection from disabling stroke, hemorrhagic stroke and cardiovascular death.
"This is certainly something that can and should be considered," says Hakan Oral, M.D., a cardiologist at the University of Michigan Frankel Cardiovascular Center — one of the first facilities in the nation to use the pioneering device. But it's not right for everyone.
How the Watchman works
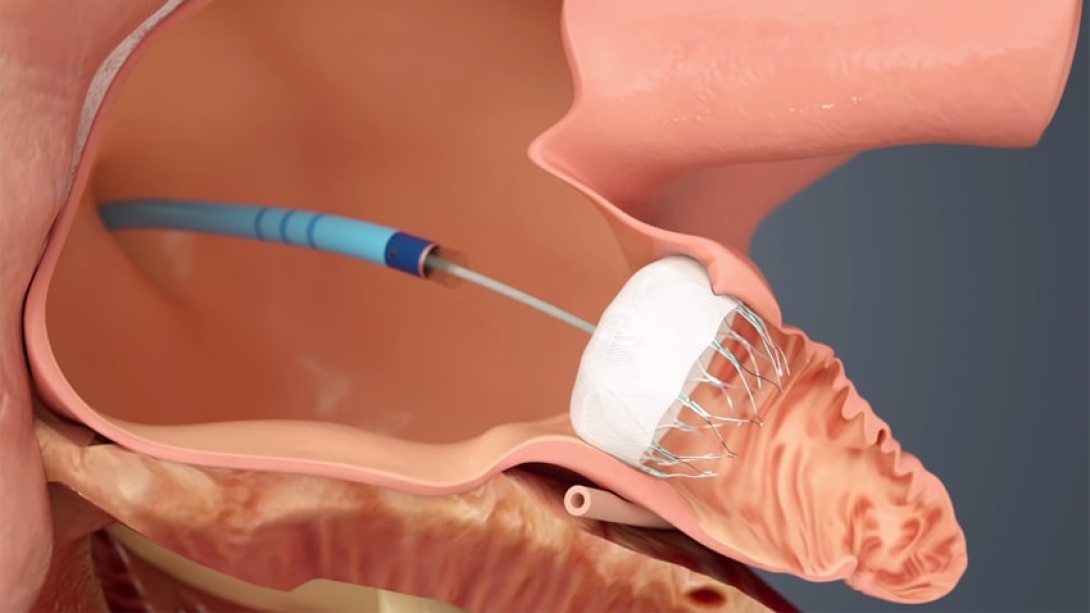
The Watchman is inserted with a wide-sheathed catheter placed through an incision in the patient's upper leg. Once in place, the self-expanding device is opened (similar to an umbrella) to seal off the heart's left atrial appendage, where harmful blood clots can form and then enter the bloodstream, thus reducing the risk of stroke and other systemic embolization.
The device bears a surface covered with a thin, permeable filter, allowing blood to pass through while simultaneously stopping clots. A patient's own heart tissue will grow over the permanent implant in time.
The Watchman comes in several sizes (as large as 33 millimeters) to accommodate individual left atrial appendages.
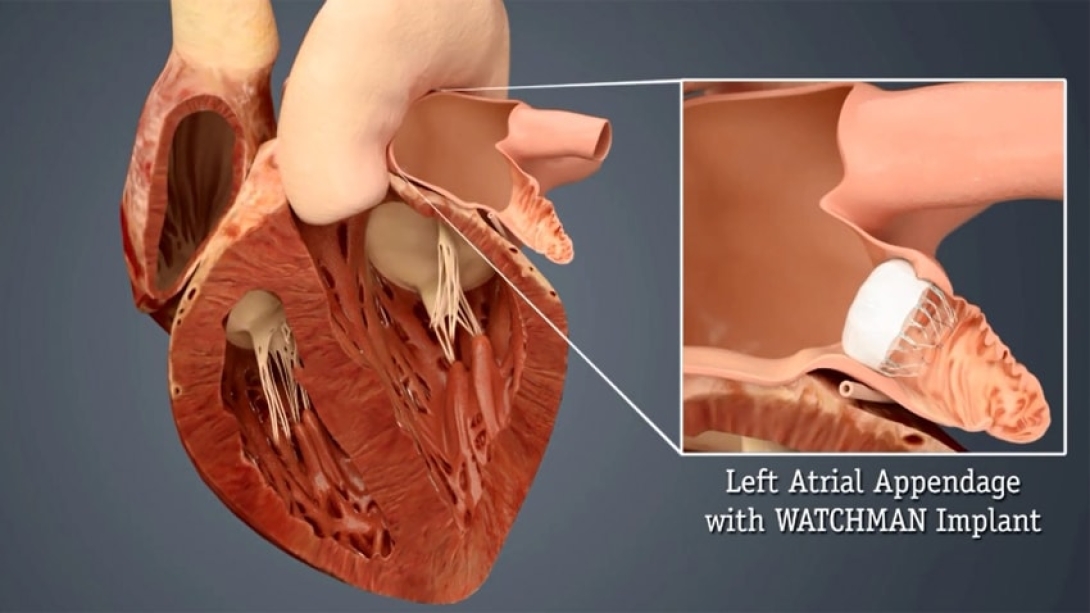
Who is a candidate?
A team of specialists determines whether patients are eligible for the device.
According to the Food and Drug Administration (PDF), the Watchman is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who:
-
Are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy;
-
Are deemed by their physician(s) to be suitable for warfarin; and
-
Have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared with warfarin.
Despite the Watchman's inconspicuous size and relative ease of insertion with general anesthesia and, typically, one night of recovery in the hospital, it isn't appropriate for everyone.
For example, those who can take warfarin without incident or risk, Oral says, need a compelling reason to receive the implant.
"If a patient has a high risk of bleeding while on blood thinners, then it is a very good option," he notes, adding that general risks of taking such medication increases with age. In turn, that makes certain older patients suitable candidates.
Weaning off warfarin
Although one of the goals of implanting the Watchman is to help wean an individual off warfarin, the drug will remain a part of the patient's regimen for 45 days after the Watchman is inserted. After that, clinicians will use a transesophageal echocardiogram to determine if the implant has successfully closed the opening of the left atrial appendage.
"If all goes well, the patient is taken off of warfarin and put on a different type of blood thinner known as Plavix, along with aspirin, for another four and one-half months," says Oral.
At that point, the patient stops taking Plavix and continues with an aspirin regimen. The Watchman, then, will keep watch on its own.

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!