The novel immunotherapy had increased rates of desensitization to peanut.
9:33 AM
Author |
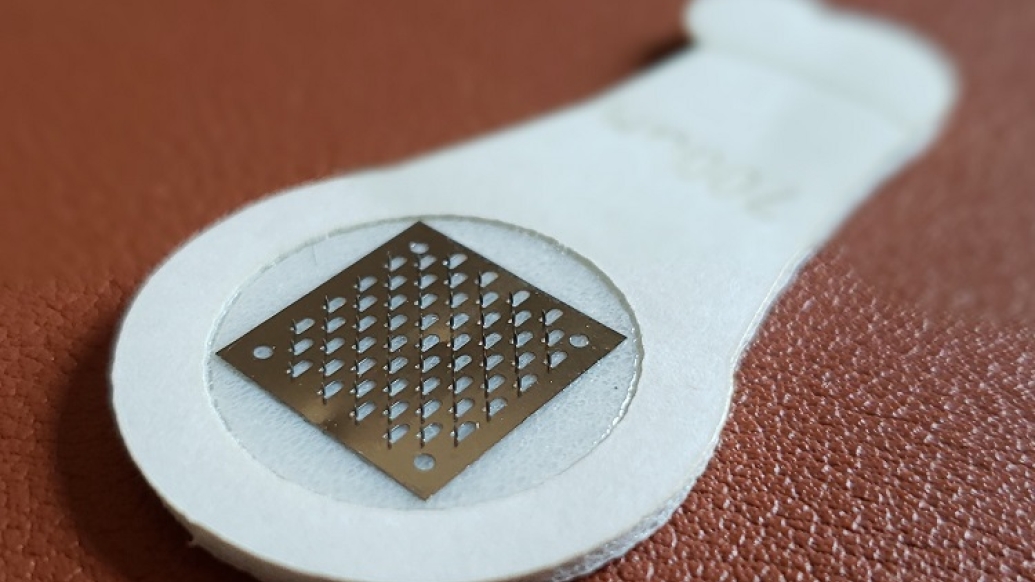
Treating peanut allergy with microneedles could significantly improve desensitization by directly targeting the allergen to the skin, providing greater protection from severe allergic reactions for millions of people, a new study suggests.
Investigators at Michigan Medicine, in collaboration with researchers from Moonlight Therapeutics, tested a dermal stamp containing peanut-coated microneedles on mice by applying it to the skin for five minutes once a week over five weeks. They compared that to mice receiving epicutaneous immunotherapy, which involves wearing a patch on the skin for 24 hours over the same time period.
The results, published in Immunotherapy, reveal mice that received the five weekly microneedle treatments had significant increased rates of desensitization to peanut allergy compared with EPIT, which required two months of treatment to achieve protection. The microneedle treatment success was achieved despite applying a dose of peanut protein 10 times lower than the dose delivered by EPIT.
"While our pre-clinical results are from studies in animal models, they demonstrate the potential for peanut microneedles to improve food allergen immunotherapy through the skin," said Jessica O'Konek, Ph.D., senior author of the paper and research assistant professor at the Mary H. Weiser Food Allergy Center at Michigan Medicine. "Treatment options for food allergy are limited, so there is a lot of motivation for the development of novel therapeutics. It will be exciting to watch the clinical development of this technology," she said.
SEE ALSO: Bone Marrow Disorder Nearly 10-Times More Common in Those with Venom Allergy
Around 6 million Americans have a peanut allergy, with symptoms that can range from mild hives to potentially fatal anaphylactic reactions. Currently, orally administered immunotherapy is the only treatment for peanut allergy approved by the United States Food and Drug Administration. However, it requires that patients follow a strict long-term protocol for ingesting each dose.
EPIT has been demonstrated to be safe in clinical trials, but the treatment showed variability in efficacy. O'Konek believes this could be due to the barrier provided by the skin surface, which may limit the amount of allergen taken up by the body. Targeted delivery of peanut protein with microneedle patches may offer more controlled delivery of allergen.
"This is a very interesting technology that could provide a unique method to desensitize people with food allergies," said James R. Baker, Jr., M.D., co-author of the paper and director of the Mary H. Weiser Food Allergy Center. "These successful animal results argue for further development of this platform."
Like Podcasts? Add the Michigan Medicine News Break on iTunes, Google Podcasts or anywhere you listen to podcasts.
Additional authors include: Mary H. Weiser Food Allergy Center: Jeffrey Landers, Katarzyna Janczak; Akhilesh Kumar Shakya & Harvinder Singh Gill, Texas Tech University Department of Chemical Engineering; Vladimir Zarnitsyn & Samirkumar R. Patel, Moonlight Therapeutics, Inc.
The microneedle device used in the study was based on the proprietary treatment platform TASIS (Targeted Allergen-Specific Immunotherapy within the Skin) developed by Atlanta-based Moonlight Therapeutics.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases under award numbers R42AI143011 and R01AI135197. The content does not necessarily represent the official views of the National Institutes of Health.
Gill and Shakya are co-inventors on a patent related to coated microneedles for allergen immunotherapy. Moonlight Therapeutics is pursuing this technology for developing microneedles for peanut and other food allergy immunotherapies. Gill and Shakya have equity in Moonlight Therapeutics. Patel and Zarnitsyn are employees of Moonlight Therapeutics and have equity in the company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Paper cited: Targeted allergen-specific immunotherapy within the skin improves allergen delivery to induce desensitization to peanut," Immunotherapy. DOI: 0.2217/imt-2021-0206
Like Podcasts? Add the Michigan Medicine News Break on iTunes, Google Podcasts or anywhere you listen to podcasts.

Explore a variety of health care news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!





