Most human oocytes never get a chance to mature into eggs — a new study sheds light on why
11:00 AM
Author |

A new 'atlas' of the human ovary provides insights into how healthy eggs develop and hormones are produced. It could lead to new research to restore ovarian endocrine function and the ability to have biologically related children, according to University of Michigan researchers.
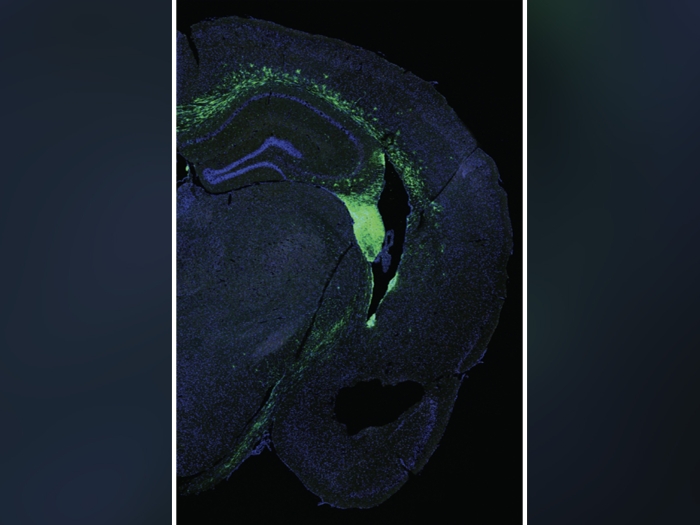
Utilizing new genetic tools, U-M’s team was able to measure the activity of most genes in many locations in the ovary — particularly around the follicles, which produce hormones and carry the immature precursors of eggs, called oocytes. The work revealed the factors that enable oocytes to mature into healthy eggs.
This new map of the ovary, reported in Science Advances, provides a deeper understanding of how oocytes interact with the surrounding cells during the normal maturation process, and how the function of the follicles may break down in aging or fertility related diseases. Such knowledge may also accelerate the development of artificial ovaries.
Follicle survival
Currently, surgeons can implant previously frozen ovarian tissue — stored before exposure to toxic medical treatments such as chemotherapy and radiation for cancers — to temporarily restore hormone production and egg maturation. However, this does not work for long because so few follicles survive through reimplantation, the researchers say.
Given the small size of the follicles, a spatially resolved analysis down to a few tens of microns is required to learn the location and function of the key cells involved in maturing the oocyte. Spatial transcriptomics allowed researchers to read strands of RNA, which are like notes taken from the DNA strand, revealing which genes are being activated in a local area. Working with an organ procurement organization, the team performed single cell RNA sequencing of and spatial analyses on ovaries from five young donors without prior disease in the ovary.
"Now that we discovered dozens of new genes that are specifically expressed in the oocytes and supporting cells, we can test whether affecting these genes could result in creating a functional follicle,” said Ariella Shikanov, an associate professor of biomedical engineering, who co-led the study with Jun Z. Li, professor of human genetics.
“This can be used to create an artificial ovary, producing eggs that could eventually be fertilized and transplanted back into the body."
Most important locations
During reproductive years, the majority of the follicles remain dormant in the ovary’s outer layer. Every month, a small portion of these follicles activate and migrate deeper into the ovary where they gradually mature. Only a few of them eventually produce mature eggs that get released into the fallopian tube.
"When analyzing single cells, we don’t know where they were in the tissue. With spatial analyses we can focus on cells in the most important locations," said Li, who is also associate chair for research of U-M Medical School’s Department of Computational Medicine and Bioinformatics. "This is especially helpful in the ovary, where the oocytes and supporting cells are very rare when compared to other cell populations.”
"This was the first time where we could target ovarian follicles and oocytes and perform a spatial transcription analysis, which enables us to see which genes are active and where," Shikanov said. "This new data allows us to start building our understanding of what makes a good egg — what determines which follicle is going to grow, ovulate, be fertilized and become a baby."
Engineered ovarian tissue
With the ability to guide follicle development and control the environment around them, the team believes that engineered ovarian tissue could function for much longer than unmodified tissue. This means patients would have a longer fertility window as well as a longer period in which their bodies produce hormones that help regulate the menstrual cycle and support the health of other organs, such as the heart, breasts and bone.
The team’s ability to analyze the data that tracked all of the gene activity was supported by funding from the Chan Zuckerberg Initiative. U-M researchers are part of the initiative’s Female Reproductive System Seed Networks.
The study is part of the Human Cell Atlas project, which seeks to “map every cell type in the human body to transform understanding of health and disease." The new spatial technology adopted by the team, NanoString GeoMx, was established in U-M by the Single-Cell Spatial Analysis Program (URL: https://singlecellspatialanalysis.umich.edu/), part of the Biosciences Initiative.
The majority of the study was carried out by two graduate students: Andrea S.K. Jones, who recently graduated with a PhD in biomedical engineering, and D. Ford Hannum, PhD student of bioinformatics. Jones and Hannum are co-first authors of the study. Sue Hammoud, associate professor of human genetics, and Erica Marsh, professor of obstetrics and gynecology are also principal investigators of the U-M's Seed Network and co-authors in this paper. Additional financial support was provided by the National Institutes of Health.
Citation: Cellular atlas of the human ovary using morphologically guided spatial transcriptomics and single-cell sequencing, Science Advances, DOI: 10.1126/sciadv.adm7506
Sign up for Health Lab newsletters today. Get medical tips from top experts and learn about new scientific discoveries every week by subscribing to Health Lab’s two newsletters, Health & Wellness and Research & Innovation.
Sign up for the Health Lab Podcast: Add us on Spotify, Apple Podcasts or wherever you get you listen to your favorite shows.

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!