Comprehending how pancreatic tumor cells thrive might be the key to new therapies and diagnostic tools.
8:00 AM
Author |
The biochemical pathways and metabolic requirements that enable tumor survival and growth may be used to design targeted cancer therapies.
Costas Lyssiotis, Ph.D., studies this idea in his lab at the University of Michigan Rogel Cancer Center.
"I would argue that the most important feature about a cancer cell is its metabolism," says Lyssiotis, an assistant professor of physiology and medicine. "Normal cells can only become cancer cells by rewiring the way that they acquire and utilize nutrients, the processes generally referred to as their metabolism."
The reason that cancer cells do this is that they have two primary goals: to survive and to reproduce.
Lyssiotis explains that if you are a cell within the context of a healthy organism, you listen to the instructions provided by the body to determine when it's time to eat, when it's not time to eat, when it's time to recycle materials that have gone bad or when it's time to die.
If you're a cancer cell, on the other hand, you've gone rogue. You ignore these signals, and take up nutrients, when and as much as you want.
"But because you're a rogue cell, the rest of the body is out to get you," Lyssiotis explains. "So you're scurrying away, trying to live in other places. You wind up in nonnative environments that can be very stressful and nutrient poor. So these cells divert a lot of energy to fending off this stress. But, since they are nutrient limited, they wind up on the brink of starvation."
If researchers can understand metabolically what makes cancer cells different from normal cells, they might be able to exploit this knowledge to design better drug targets and therapies.
In other words, these cells can be pushed over the edge and starved to death.
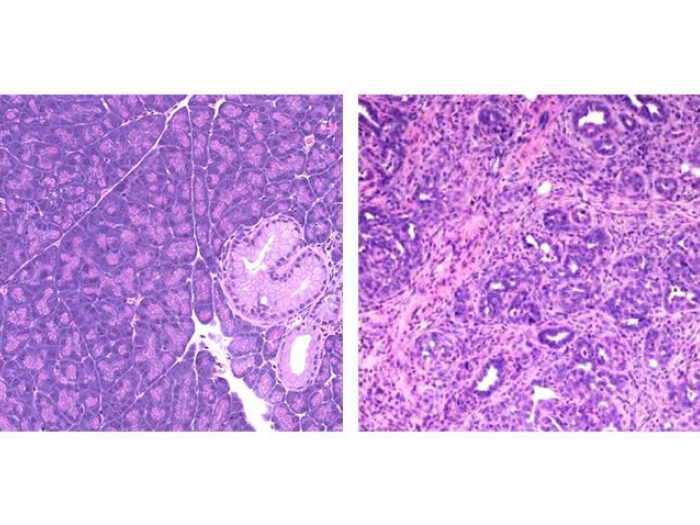
Pancreatic cancer's unique metabolism
Unlike many tumors, pancreatic tumors are rock hard. When researchers examine what's inside, the cancer cell content can be as low as about 10 percent.
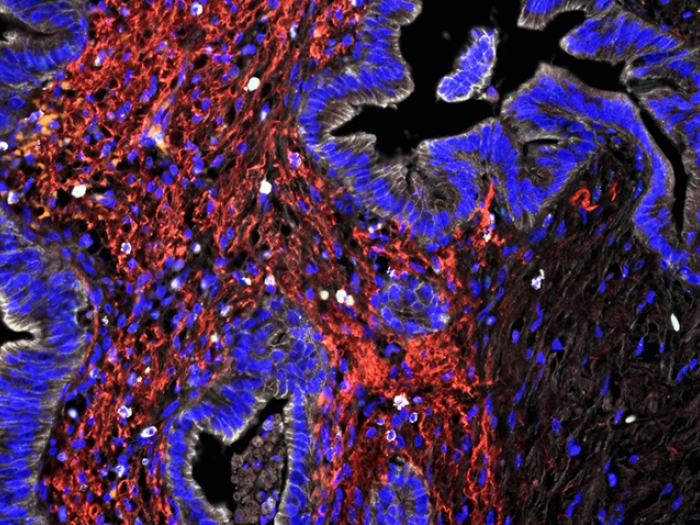
Much of the rest of the tumor is a type of scar tissue, called stroma. The stroma releases a lot of extra cellular matrix protein, which retains water, creating pressure so that the blood vessels collapse. Without vasculature, the pancreatic cancer cells are left to find nutrients outside the blood stream.
This stresses the cancer cells inside the pancreatic tumor. They can't get the nutrients and oxygen they need, which leads to oxidative stress. At the same time, the immune system is trying to figure out how to kill the cancer cells, causing additional stress. You would think with all these stressors that the cancer cells would not survive. But they do, and they kill patients.
How? Lyssiotis says pancreatic cancer cells turn into professional scavengers in a variety of ways. They activate recycling pathways within the cell, and they forage for nutrients outside the cell in nontraditional ways.
One of these processes is called macropinocytosis, where cells eat whatever they can find in the bulk extracellular space of the stroma. Researchers suspect that this leaves the remaining 90 percent of the pancreatic tumor as a foraging ground that the cancer can co-opt for nutrients.
This is a major focus of Lyssiotis's lab. By understanding how these nutrient-scavenging processes are activated and used, Lyssiotis and his colleagues can perhaps identify new selective and safe drug targets.
Cancer cells use different metabolic pathways than normal cells, and understanding how these pathways work is important for identifying [new] drug targets.Costas Lyssiotis, Ph.D.
Exploiting pancreatic cancer's unique characteristics
Metabolomics, a technique that allows for the study of all the metabolic reactions inside the cell at the same time, is a key technology in this research.
"This information gives us a sense of the metabolism pathways that are being used by the cells," Lyssiotis says. "Cancer cells use different pathways than normal cells, and understanding how these pathways work is important for identifying drug targets that when inhibited with a cancer-targeted drug will not harm the normal, healthy cells."
One of the enzymes Lyssiotis studies closely is called aspartate transaminase, or GOT1.
"As best we can tell, pancreatic cancer cells are uniquely dependent on the action of this enzyme, and normal cells tolerate inhibition without overt consequence," says Lyssiotis. "Recently, we have teamed up with a pharmaceutical company to develop drugs that inhibit GOT1. These will be moved to preclinical development in the near future."
But don't expect the drugs to work alone. Few targeted drugs will, Lyssiotis says.
"Cancer cells ultimately find a pathway of resistance to a single drug. Logical combinations of drugs are harder for cancer cells to block quickly," he explains. "That understanding is the foundation for precision medicine."
Further prospects in cancer research
Lyssiotis says new imaging techniques based on cell metabolism are also on the horizon. These could help detect disease earlier, potentially finding pancreatic tumors when surgery is still an option.
The technique could have implications for other types of cancer as well. It's all about tailoring cancer diagnosis and treatment to each individual.
"Precision medicine is coming into its own," says Lyssiotis. "There's an electricity, a cumulative appreciation that this is the way to go, that we need a better, a deeper understanding of each individual's tumor if we are going to treat patients effectively. I believe cell metabolism is going to play a big role in that."

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!