How a visualization — and a new understanding — of a class of “defender proteins” may help scientists fight HIV infection.
7:00 AM
Author |
When HIV infects a patient, it doesn't just attack the person's body. The virus inserts a copy of its genetic material into the genome — hijacking the patient's biological machinery to "hide out" until conditions are right to replicate.
MORE FROM THE LAB: Subscribe to our weekly newsletter
"That's the reason an HIV infection is so difficult to cure. We do not have a way to get rid of it completely because it integrates into our genome.
"But our bodies have evolved ways to protect our genomes — and that's what we are studying," says structural biologist Janet Smith, Ph.D., senior author of a new study in Nature Communications that sheds light on a class of proteins that defend against HIV-1. These defender proteins mutate the viral genome so that even if it hides out, it cannot cause disease.
By better understanding how defender proteins work, scientists hope to devise new approaches to fighting the infection.
The defender proteins sneak into virus particles by hitching a ride on the HIV-1 genome. Like all retroviruses, HIV has a genome made of a single strand of RNA, unlike the double-stranded DNA helix of the human genome. After an HIV-1 particle enters human cells, the viral RNA is copied into DNA, which then is inserted into the human genome.
Smith's lab at the University of Michigan Life Sciences Institute has been investigating a class of defender proteins called APOBEC3s (for apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like, family 3), or A3s. Three of these proteins — A3F, A3G and A3H — are known to impede retroviruses by infiltrating the invader virions and then performing a massive mutation on the DNA copy of the HIV-1 genome.
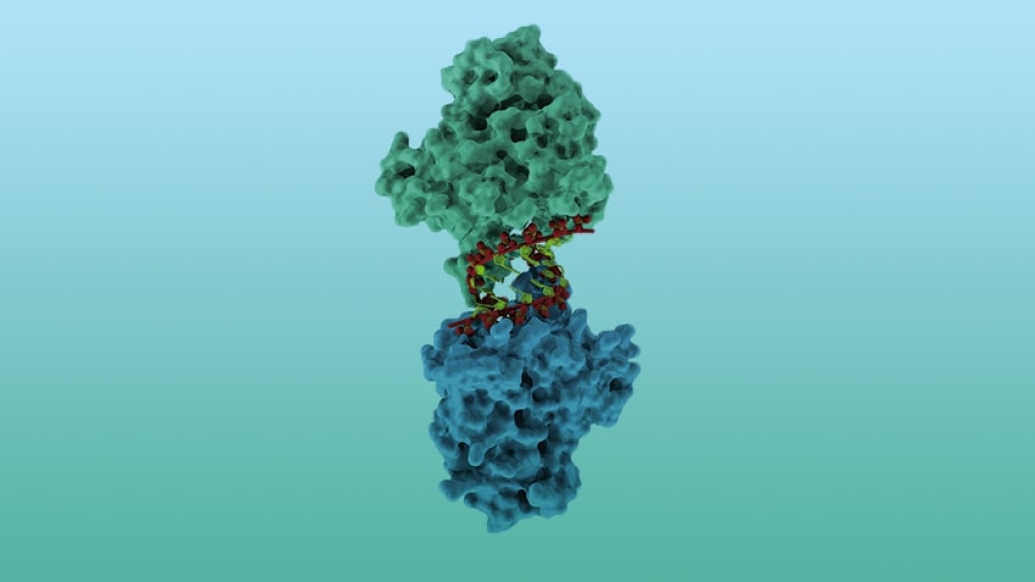
Specifically, the team of researchers led by Smith at U-M and Theodora Hatziioannou at The Rockefeller University set out to visualize the 3-D structure of A3H.
"We know that APOBEC3H slows down HIV-1," says Jennifer Bohn, a graduate student in U-M's biochemistry program and the lead study author. "We wanted to solve the structure of the protein so we can understand precisely how its structure enables this function."
Challenging assumptions
Prior to this study, researchers widely assumed that A3H first formed symmetrical pairs — what scientists call "dimers" — and then the dimers attached to single-stranded RNA in the virus. The group's findings dispel those assumptions.
SEE ALSO: The Cells That Stand in the Way of Curing HIV
Smith and her colleagues revealed that the A3H molecules do not work in conjoined pairs, nor do they bind to single-stranded RNA. Instead, two A3H molecules individually attach to opposite sides of a short double-stranded RNA helix. The Rockefeller group discovered that A3H also selects double-stranded RNA in HIV-1 virus particles.
The structural analysis offers unexpected insight into how A3 proteins recognize the foreign HIV-1 RNA as an invader, Bohn says. The RNA that gets packaged into HIV-1 virus particles is highly structured, folding and organizing in ways that create the short double-stranded helices that the A3H protein seeks out.
Once it infiltrates the virus on HIV-1 RNA, the A3H protein is in the right place at the right time to protect against the virus.
"These findings give us some clues as to how this protein utilizes RNA binding to get into the virus and how it can immediately mutate the newly formed DNA," Bohn says. "Understanding that function is crucial for understanding its ability to inhibit the virus."
"Many researchers around the world are trying to determine just how these A3 proteins work," adds Smith, who is also a professor of biological chemistry in the U-M Medical School and the associate director of the Life Sciences Institute. "In this corner of HIV research, this is a very important advance."
The study was supported by grants from the National Institutes of Health and is part of the Center for HIV/AIDS-Related Structural Biology (CRNA) based at U-M, which recently received a $25 million grant renewal from the NIH.
Other co-authors on the study are Keyur Thummar, Ashley York, Alice Raymond and Paul D. Bieniasz, of The Rockefeller University and the Howard Hughes Medical Institute, and W. Clay Brown, of the U-M Life Sciences Institute.

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!